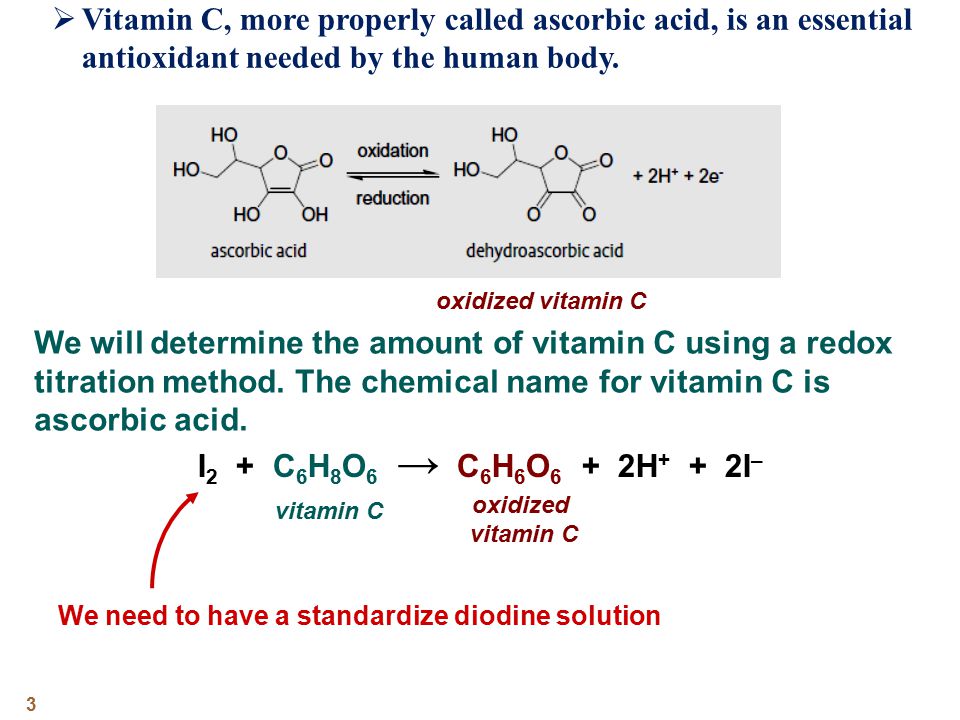
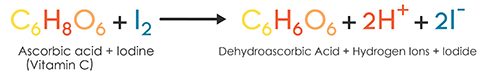
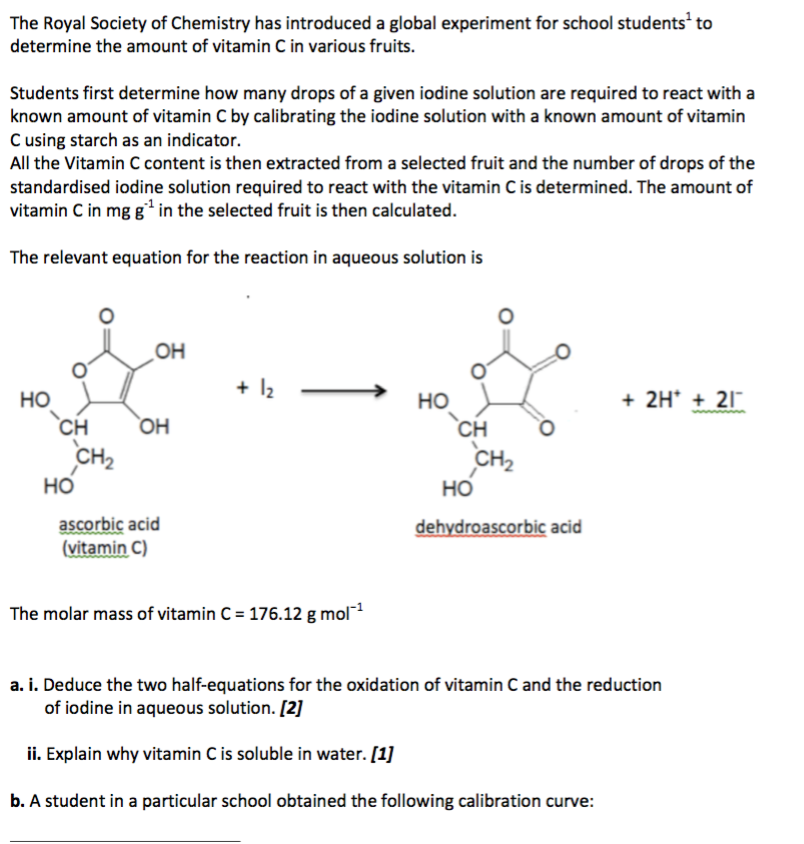
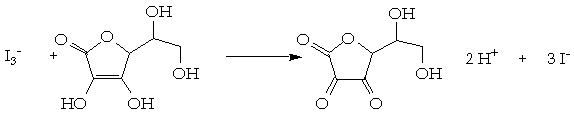
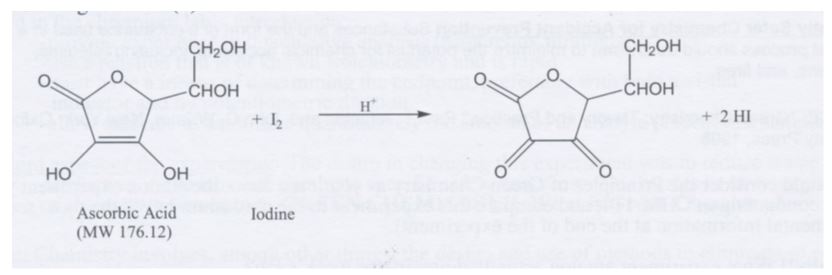
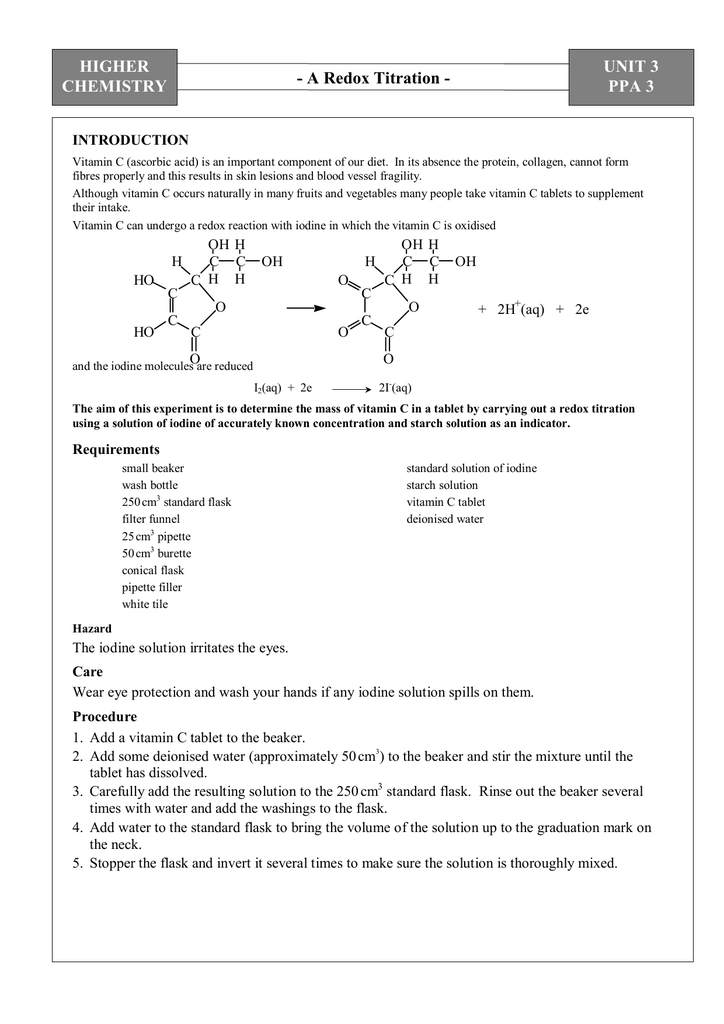
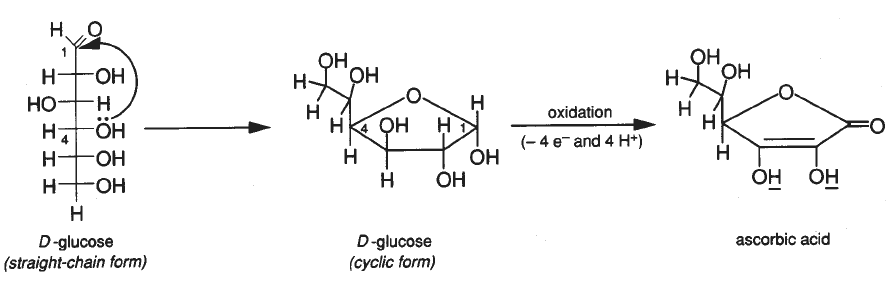
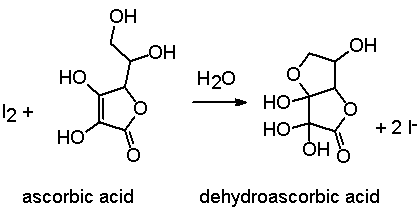
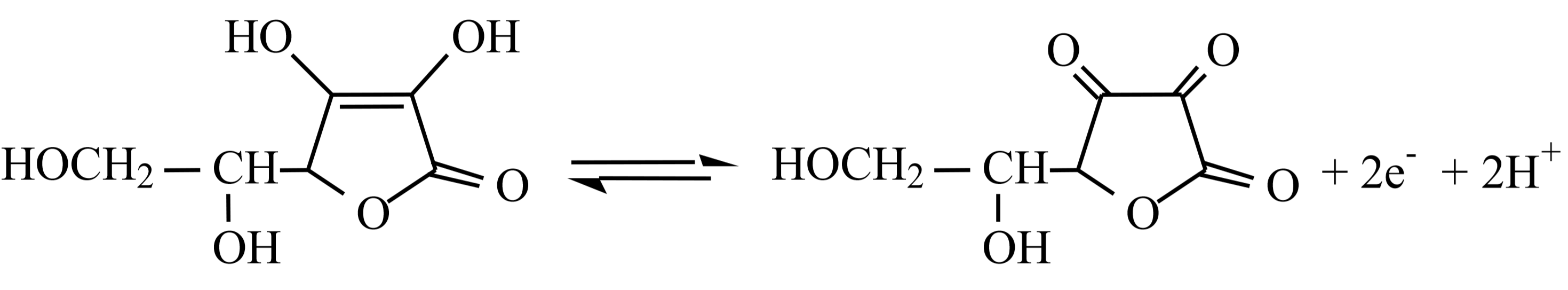
SOLVED:The amount of ascorbic acid (vitamin C) in fruit juice is determined by a titration using a redox reaction. Iodine is the titrant, but because iodine solutions in water are unstable, the
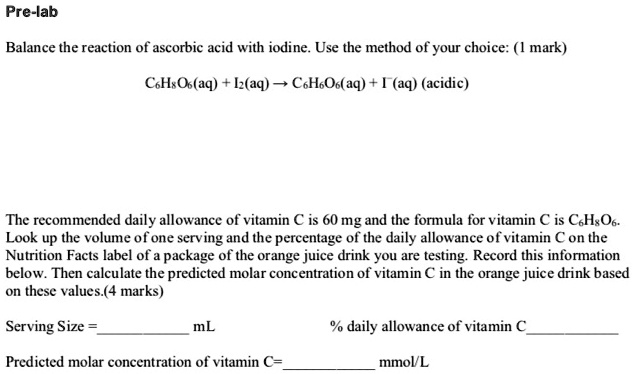
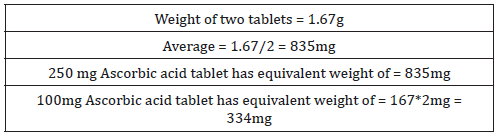
SOLVED: Pre-lab Balance the reaction of ascorbic acid with iodine. Use the method of your choice: mark) CoHsOs(aq) [z(aq) CoHsOs(aq) (aq) (acidic) The recommended daily allowance of' vitamin € is 60 mg
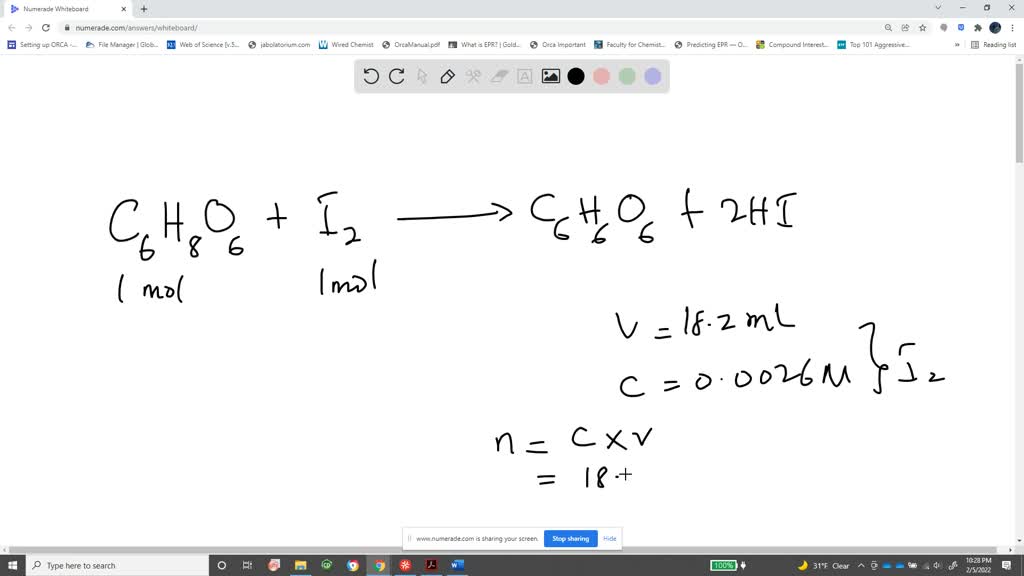
SOLVED: The titration of vitamin C with iodine proceeds according to the given equation. C6H8O6+I2⟶C6H6O6+2HI Suppose it takes 18.21 mL of 0.0026 M I2 solution to reach the end point of the