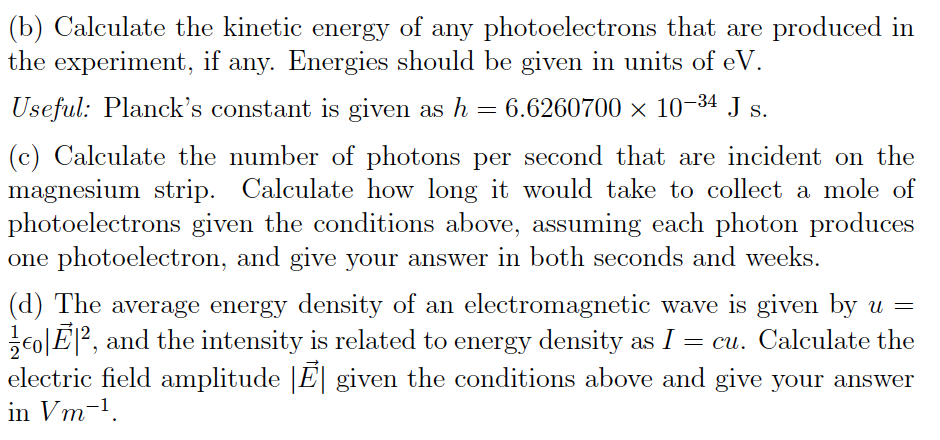
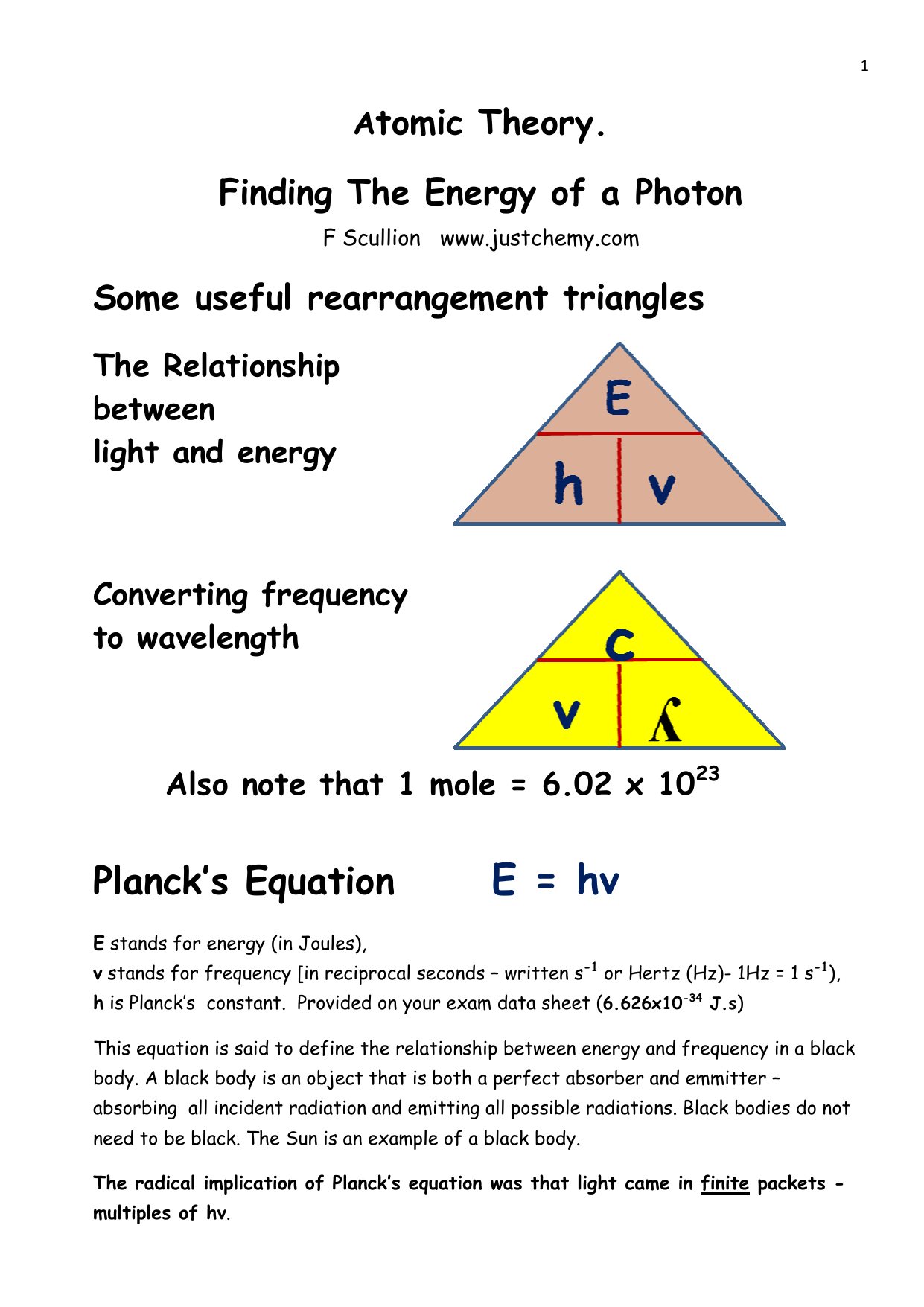
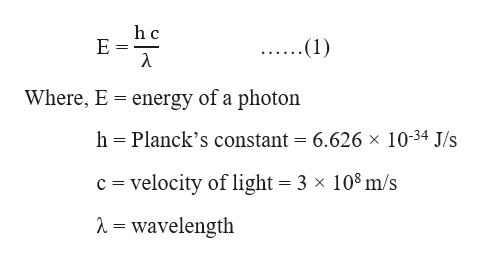
Calculate the energy in kilocalorie per mole of photons of an electromagnetic radiation having a wavelength of 7600 A.A. 33.56B. 37.56C. 47.35D. 42.35

English) Calculate the energy of one mole of photons of radiations whose frequency is 5 X 10^ 14 Hz - YouTube
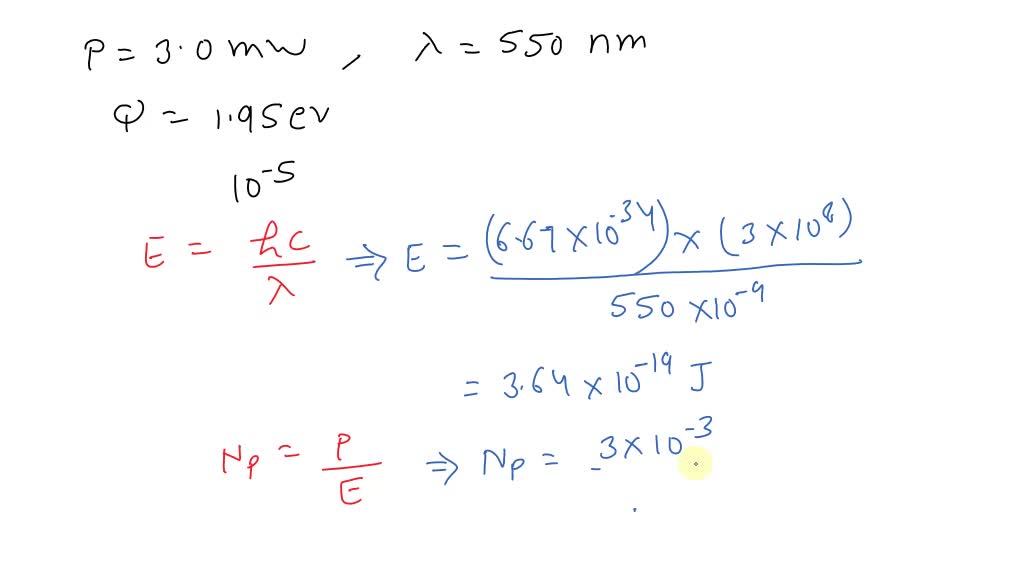
SOLVED: A radiation of 253 nm incident on HI results in the decomposition of 1·85×10 -2 mole per 1000 cals of radiant energy. Calculate the quantum efficiency.

If the photon of the wavelength 150 pm strikes an atom and one of its inner bound electrons is ejected out with a velocity of 1.5 × 10^7ms^-1 , calculate the energy
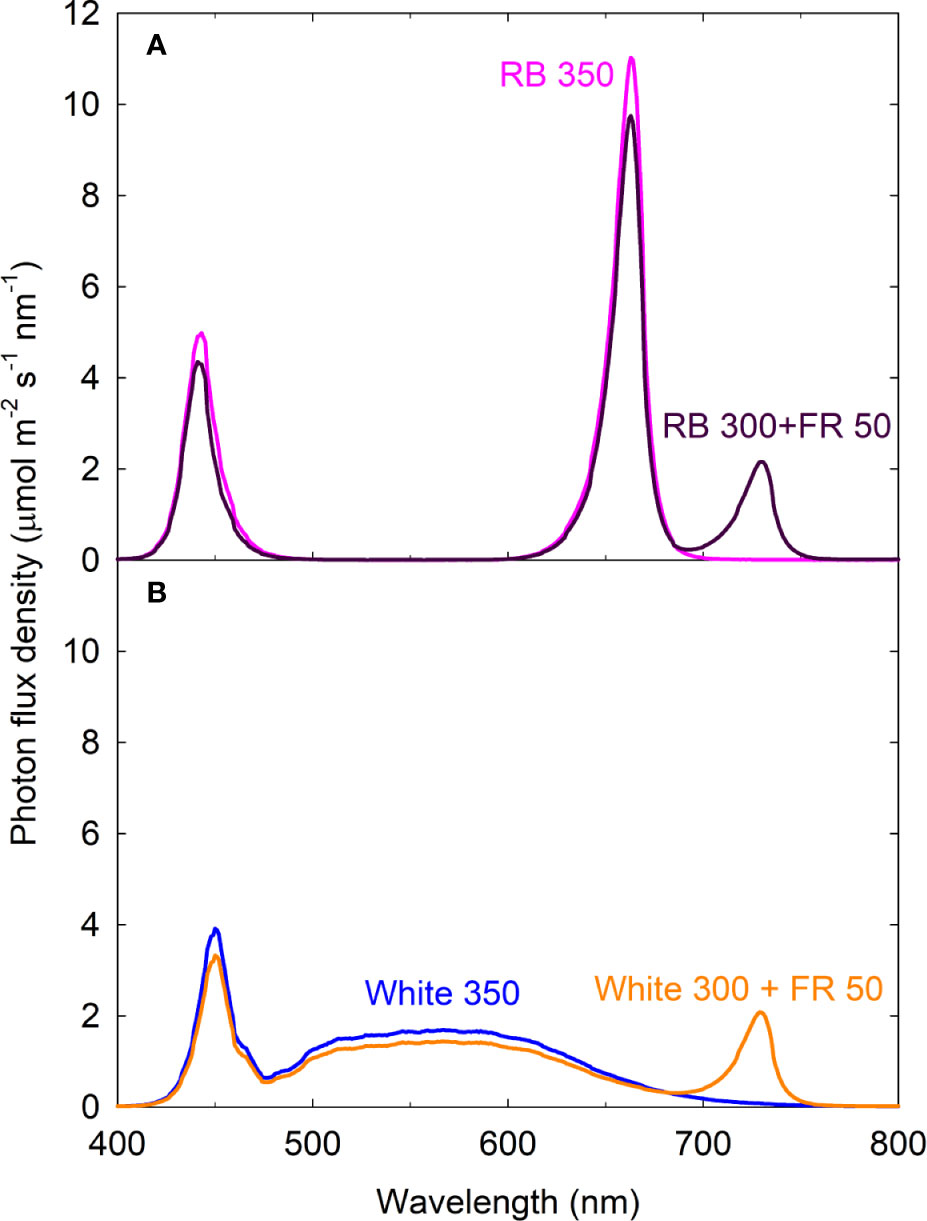
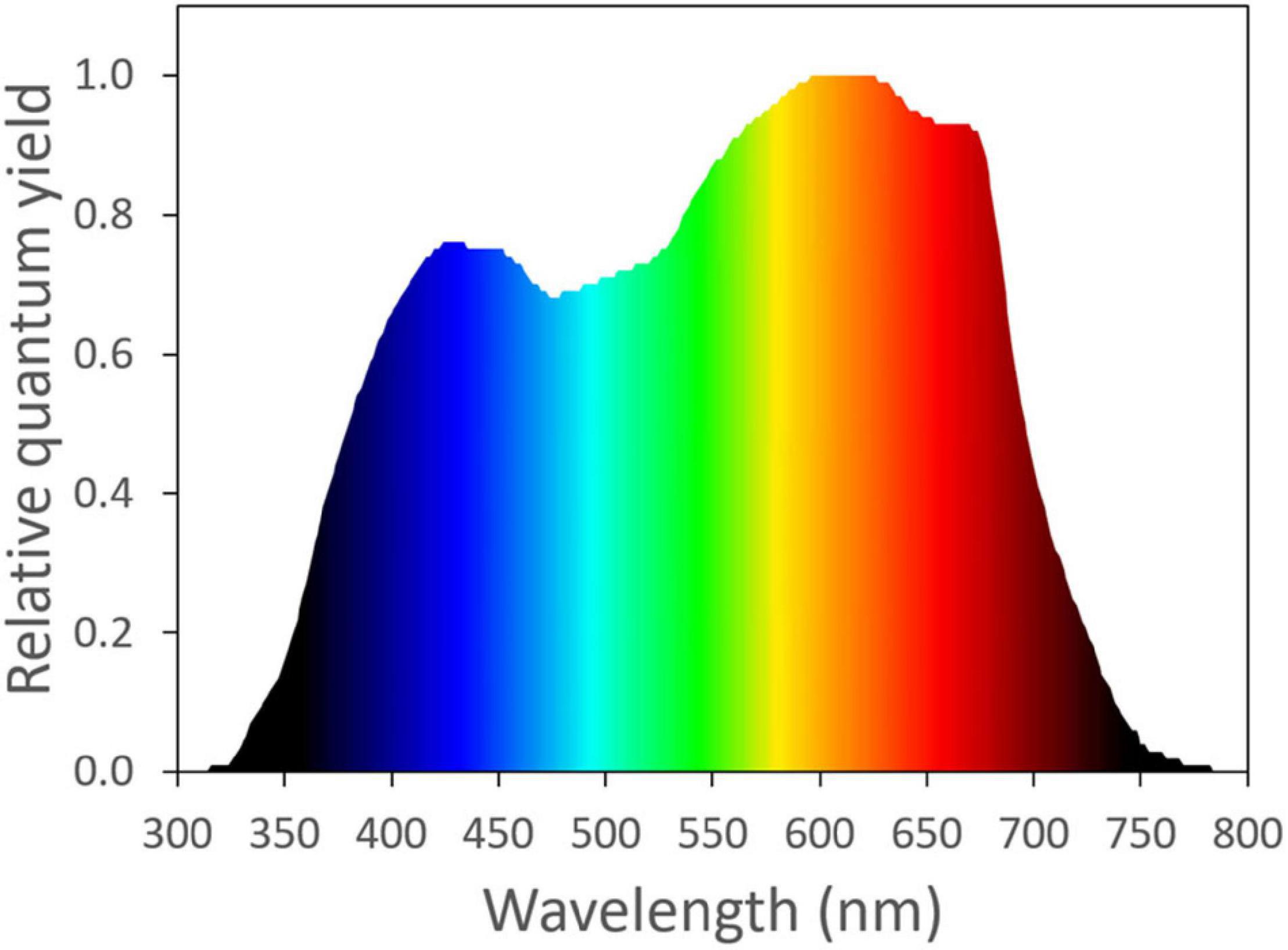
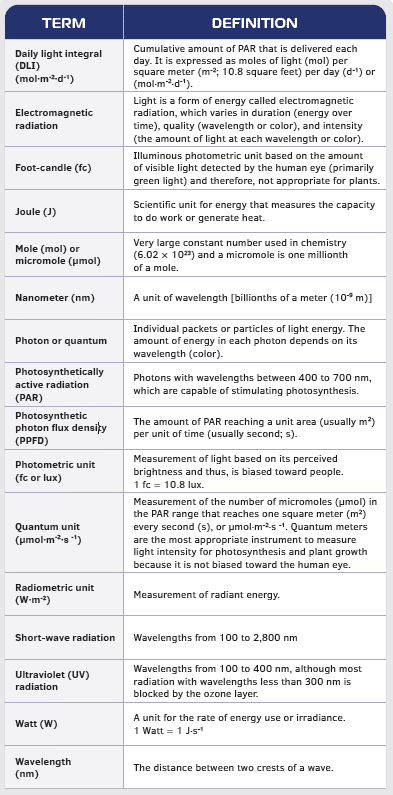
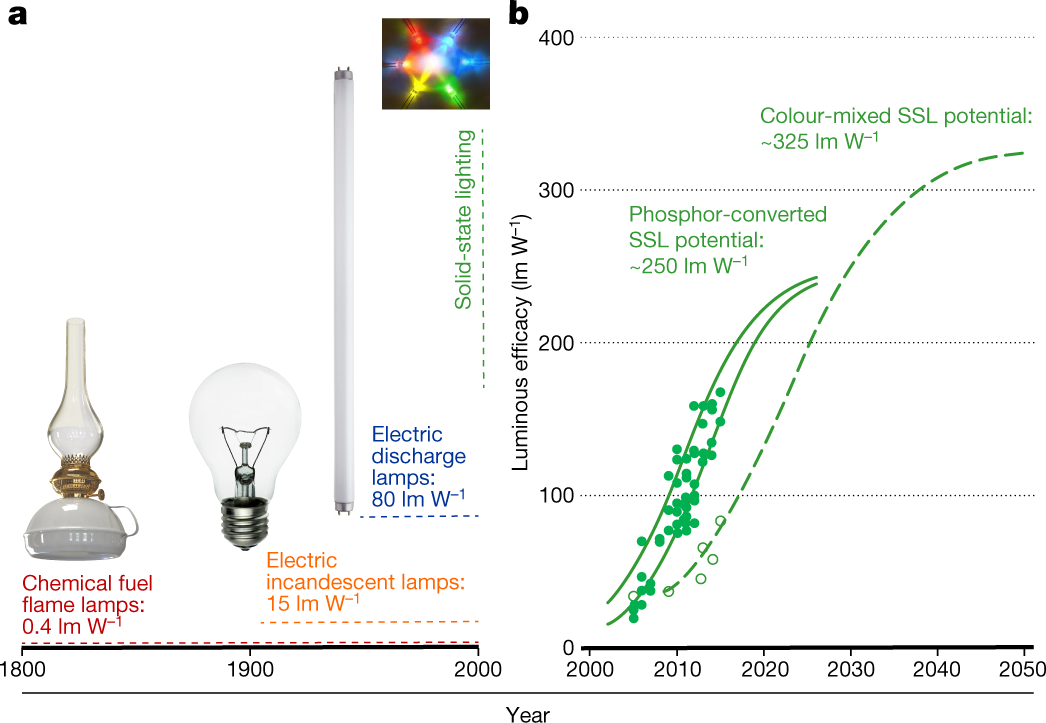
Frontiers | Substituting Far-Red for Traditionally Defined Photosynthetic Photons Results in Equal Canopy Quantum Yield for CO2 Fixation and Increased Photon Capture During Long-Term Studies: Implications for Re-Defining PAR
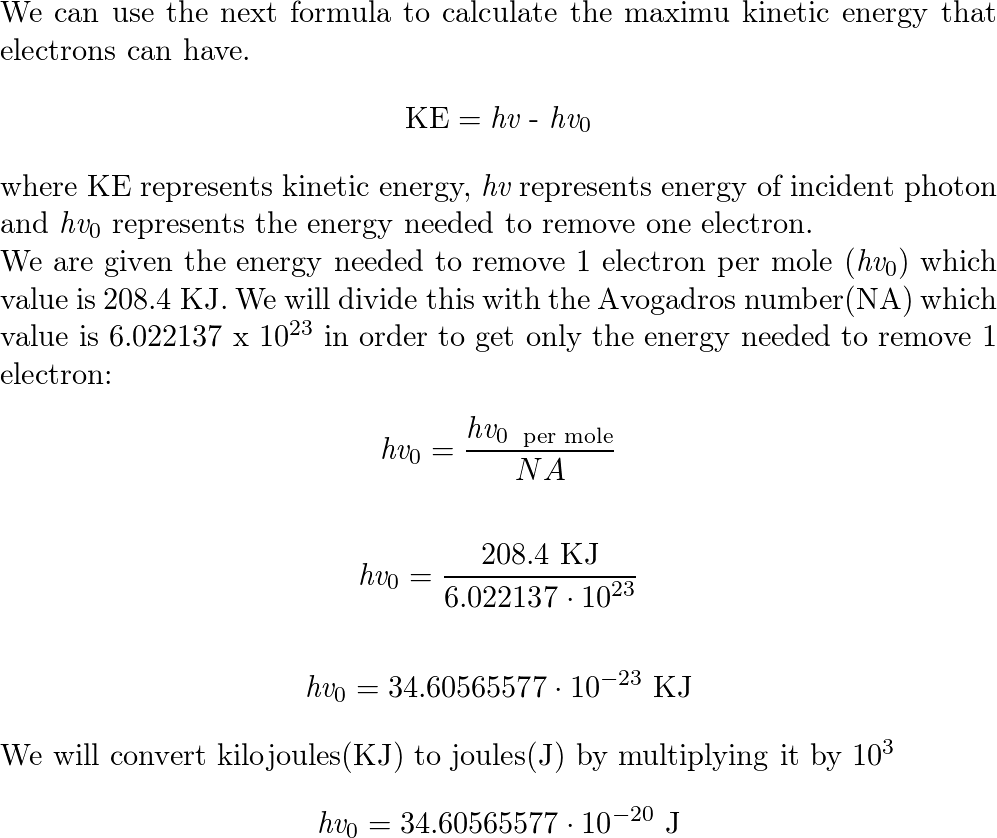
With what maximum kinetic energy will the electrons be ejected when the metal is exposed to light with a wavelength of 285 nm? - Quora

Calculate the energy of one mole of photons of radiation whose frequency is `5 xx 10^(14) Hz`. - YouTube