Specific Heat, J/g.°C 2.06 - ice 4.18 - water 2.03 - steam.Molar heat of fusion for water, kJ/mol - 6.02 - Home Work Help - Learn CBSE Forum

Calculate the change in entropy for the fusion of 1 mol of ice. The melting point of ice is 300K and molar enthalpy of fustion for ice = 6.0 k J mol^(-1).
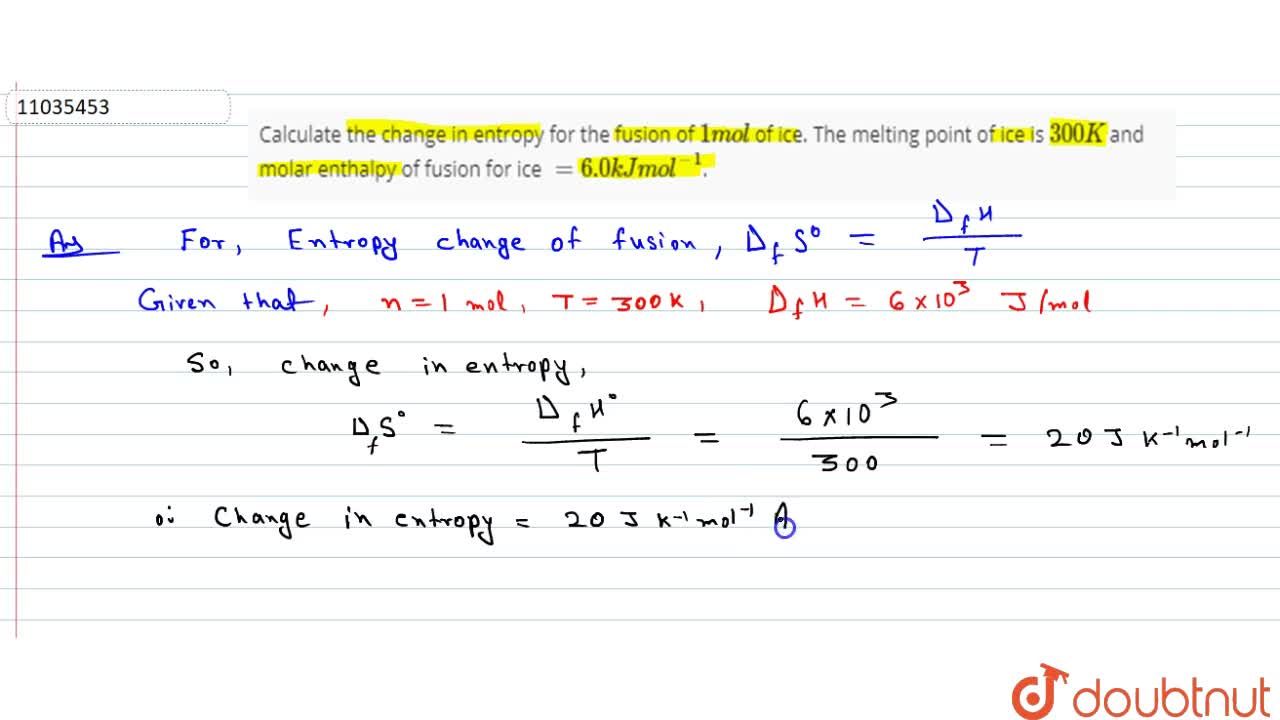
Calculate the change in entropy for the fusion of 1 mol of ice. The melting point of ice is 300K and molar enthalpy of fustion for ice = 6.0 k J mol^(-1).

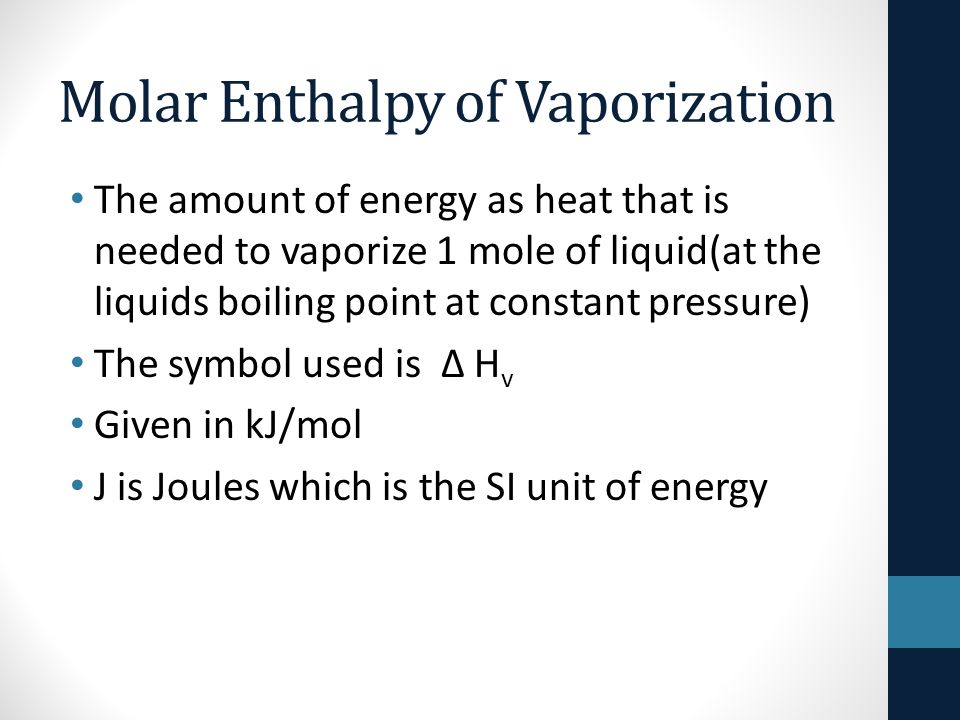
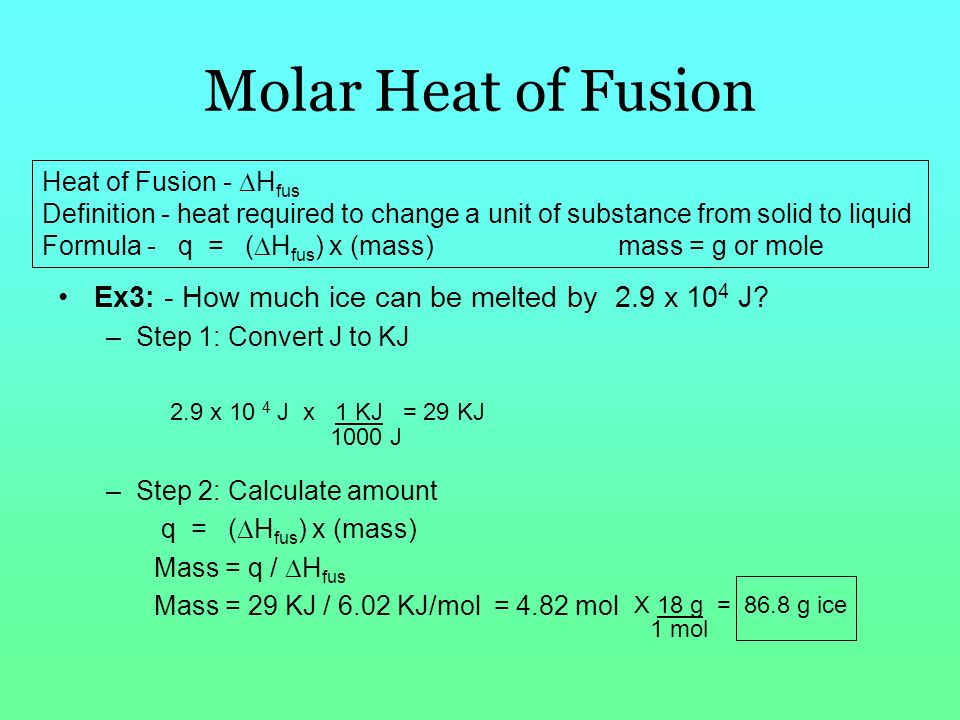
Calculations in Chapter 10. Molar Enthalpy of Fusion Used when melting or freezing = ___energy ____ mol of substance Can be arranged to find any of the. - ppt download

Calculations in Chapter 10. Molar Enthalpy of Fusion Used when melting or freezing = ___energy ____ mol of substance Can be arranged to find any of the. - ppt download
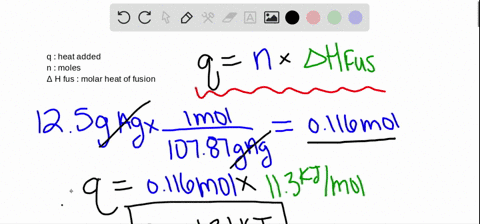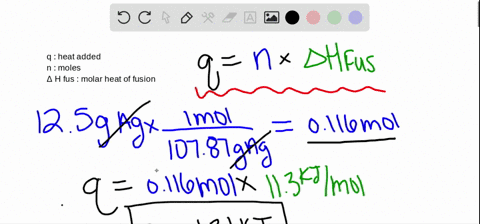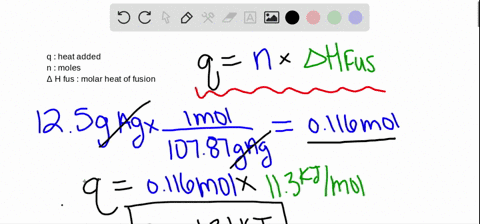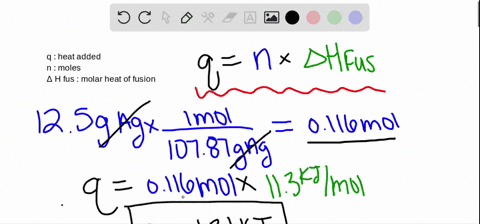
SOLVED:The molar heat of fusion of benzene is 9.92 kJ / mol . Its molar heat of vaporization is 30.7 kJ / mol . Calculate the heat required to melt 8.25 g

SOLVED: 35 The molar enthalpy of fusion of water is 6.03 kJ mol-1 at 273K and the molar heat capacities of liquid water and ice are 75.3 J K-1 mol-" and 37.9

Specific Heat, J/g.°C 2.06 - ice 4.18 - water 2.03 - steam.Molar heat of fusion for water, kJ/mol - 6.02 - Home Work Help - Learn CBSE Forum