What will be the theoretical value of spin only magnetic field when Fe(SCN)3 reacts with the solution containing F ions to yield a complex
Which of the following ion has maximum magnetic moment ? 1.Mn^3+ 2.Cu^2+ 3. Fe^3+ 4.v^3+ Also explain what is magnetic moment ?
![The spin-only magnetic moment of `[Fe(NH_(3))_(6)]^(3+) and [FeF_(6)]^(3-)` (in units of BM ) - YouTube The spin-only magnetic moment of `[Fe(NH_(3))_(6)]^(3+) and [FeF_(6)]^(3-)` (in units of BM ) - YouTube](https://i.ytimg.com/vi/KVj56QvOV1I/maxresdefault.jpg)
The spin-only magnetic moment of `[Fe(NH_(3))_(6)]^(3+) and [FeF_(6)]^(3-)` (in units of BM ) - YouTube
Amongst the following ions which one has the highest magnetic moment value? (i) [Cr(H2O)6]3+ (ii) [Fe(H2O)6]2+ - Sarthaks eConnect | Largest Online Education Community
![The spin-only magnetic moment of [Fe(NH(3))(6)]^(3+) and [FeF(6)]^(3-) (in units of BM ) respectively are The spin-only magnetic moment of [Fe(NH(3))(6)]^(3+) and [FeF(6)]^(3-) (in units of BM ) respectively are](https://d10lpgp6xz60nq.cloudfront.net/ss/web/1263733.jpg)
The spin-only magnetic moment of [Fe(NH(3))(6)]^(3+) and [FeF(6)]^(3-) (in units of BM ) respectively are

Illustration of the net magnetic moment calculation in various types of... | Download Scientific Diagram
which ion has maximum magnetic moment? 1)Fe^3+ 2)Mn^2+ and why because both has same no of unpaired electron
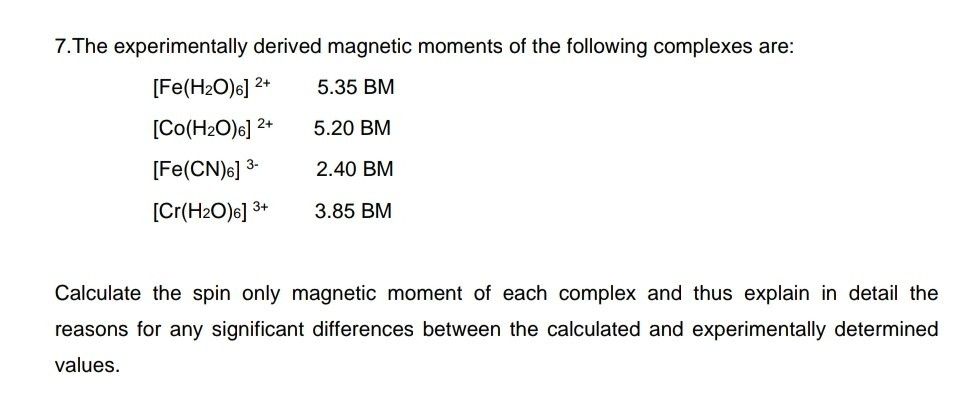
Calculate the magnetic moments of the following complexes : (i) [Fe(CN)6]-4 (ii) [FeF6]-3 - Sarthaks eConnect | Largest Online Education Community
![Explain why [Fe(H(2)O)(6)]^(3+) has magnetic moment value of 5.92 BM where as [Fe(CN)(6)]^(3-) has a value of only 1.74 BM ? Explain why [Fe(H(2)O)(6)]^(3+) has magnetic moment value of 5.92 BM where as [Fe(CN)(6)]^(3-) has a value of only 1.74 BM ?](https://d10lpgp6xz60nq.cloudfront.net/ss/web/584381.jpg)
Explain why [Fe(H(2)O)(6)]^(3+) has magnetic moment value of 5.92 BM where as [Fe(CN)(6)]^(3-) has a value of only 1.74 BM ?
![Explain why [Fe(H 2 O) 6 ] 3+ has high magnetic moment value of 5.92 BM whereas magnetic moment of [Fe(CN) 6 ] 3– has value of only 1.74 BM. Explain why [Fe(H 2 O) 6 ] 3+ has high magnetic moment value of 5.92 BM whereas magnetic moment of [Fe(CN) 6 ] 3– has value of only 1.74 BM.](https://static.insightsonindia.in/ncertusercontent/solutions/?domain=gF&l=PROJ38513/156889677077182.jpg)


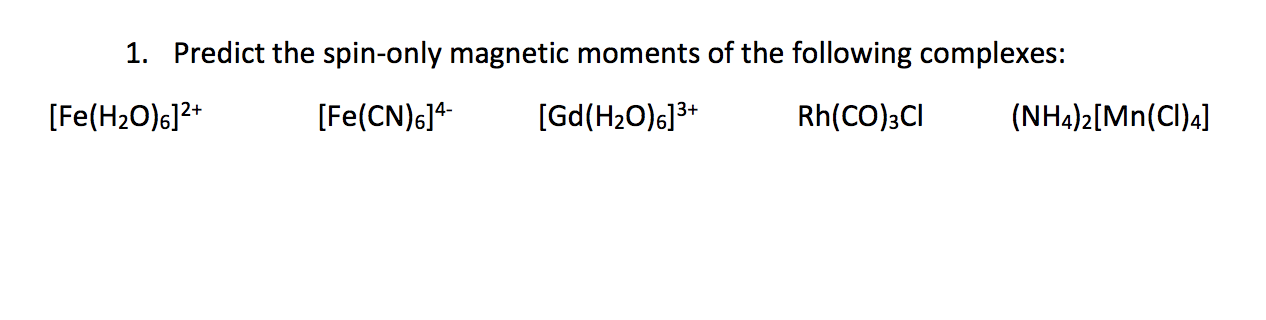








![The spin magnetic moment of iron in `K_(3)[Fe(CN)_(6)]` - YouTube The spin magnetic moment of iron in `K_(3)[Fe(CN)_(6)]` - YouTube](https://i.ytimg.com/vi/m2ePFFONgdA/maxresdefault.jpg)