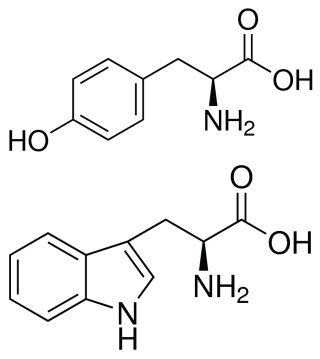
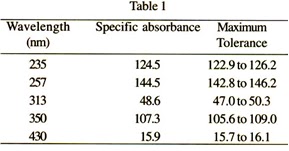
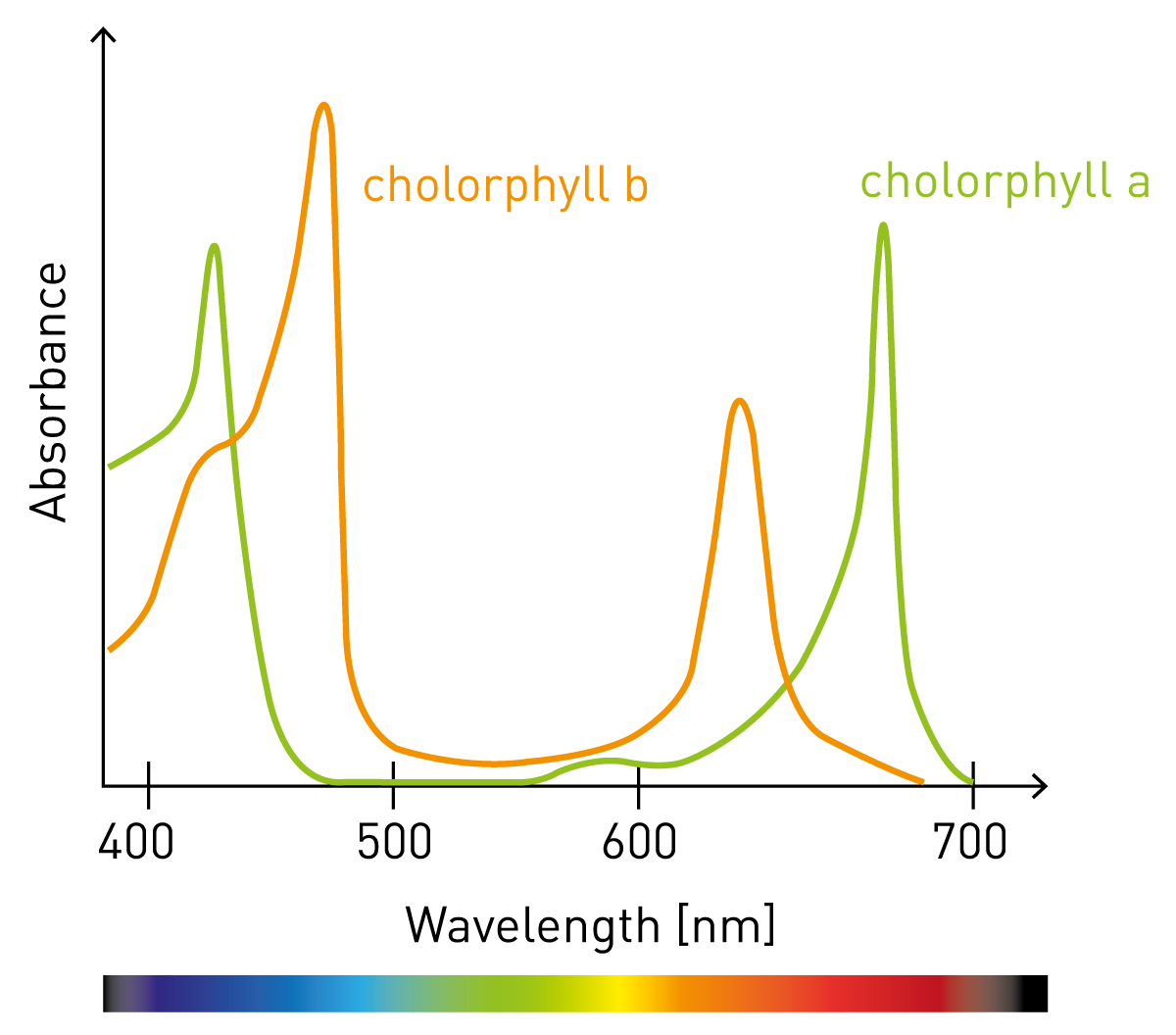
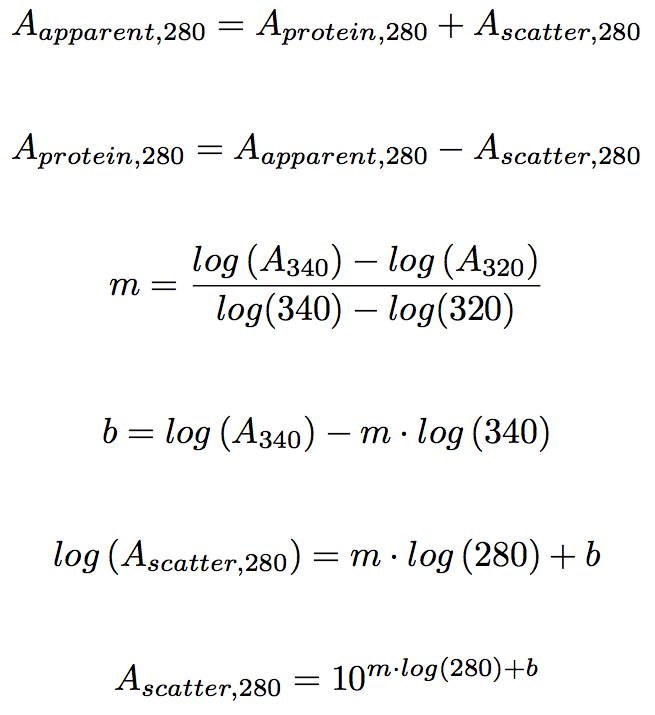
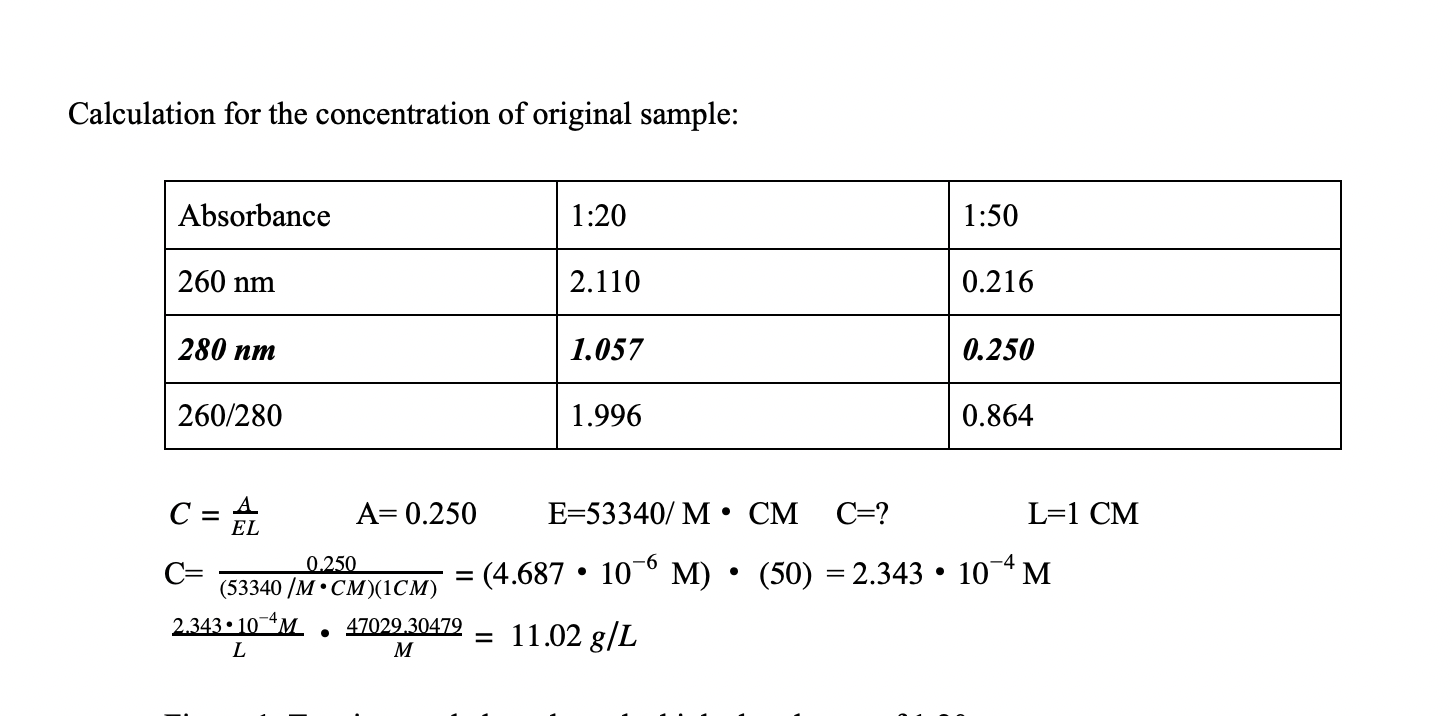
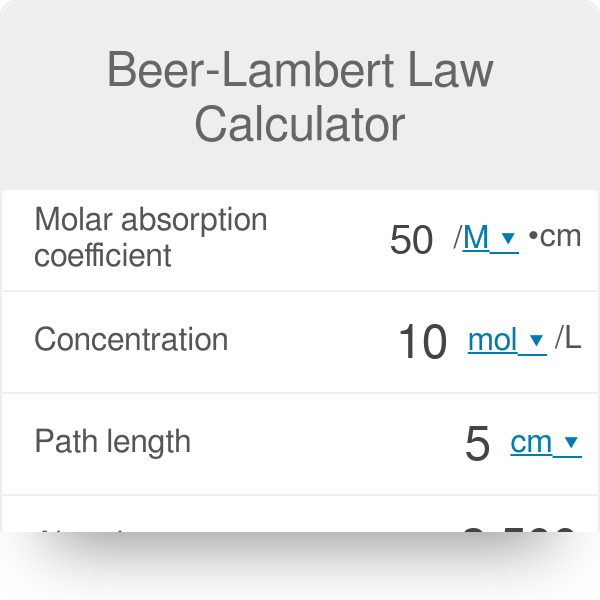
Absorbance Measurements – the Quick Way to Determine Sample Concentration - Eppendorf Handling Solutions

How to calculate the assay in UV-Vis spectrophotometer, if specific absorbance knows? | ResearchGate

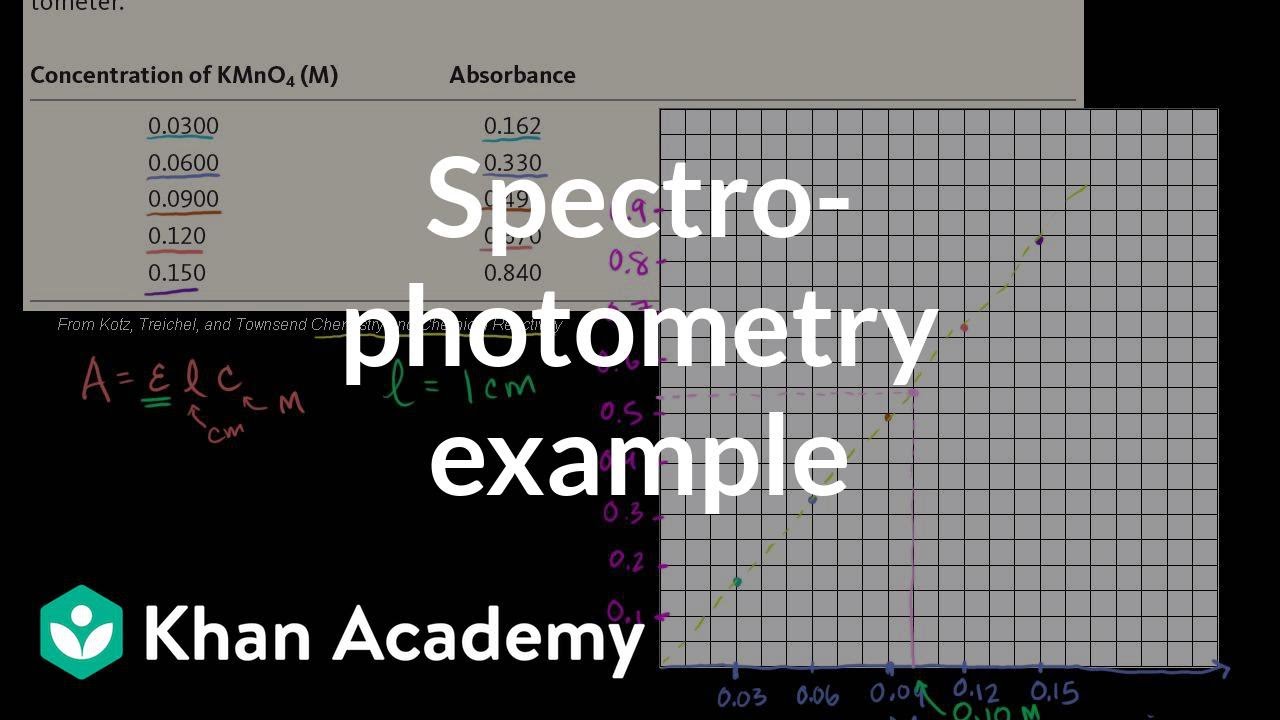
SOLVED: A spectrophotometric assay was t0 bz canied out on compound with concentration 5.04 ppm and molar absorptivity 9.60 10' L mol ! cm - Calculate the percent transmittance if the measurement


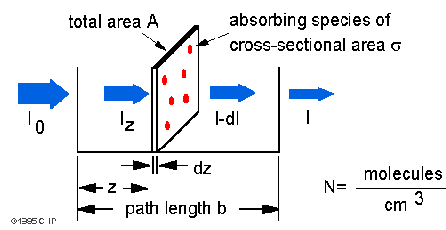
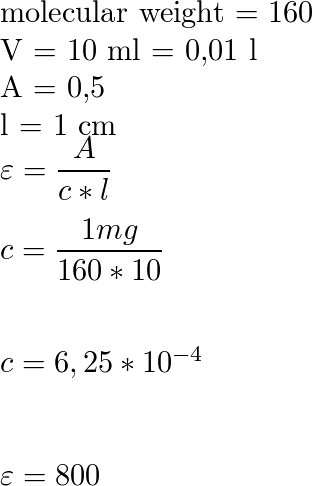
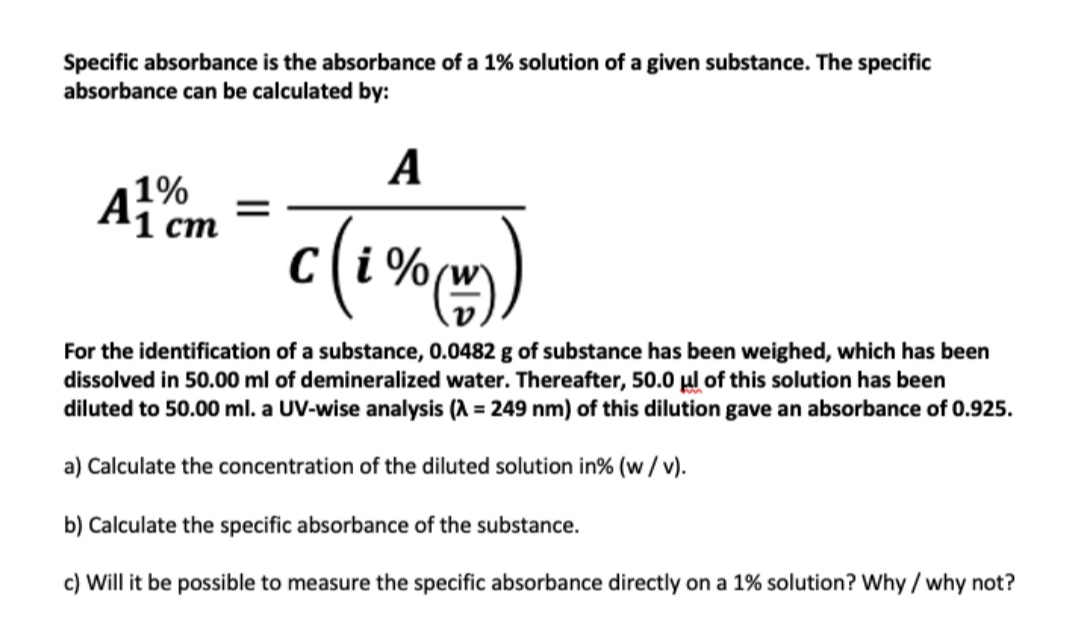

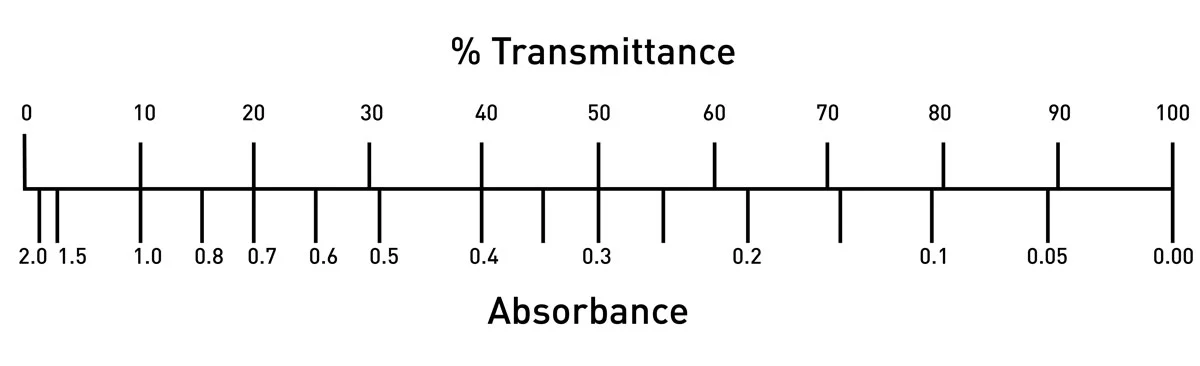
![Solved [8 marks] The concentration of chemical solutions can | Chegg.com Solved [8 marks] The concentration of chemical solutions can | Chegg.com](https://media.cheggcdn.com/media/964/96422413-45fd-4286-ae08-f3bb9f8cebd7/php5wTrL4)